2013-11-26
2013-11-24
Saranan Kepada Calon Kimia SPM
- Baca soalan dengan teliti dan fahami kehendak tugasan.
- Mesti merancang sebelum menulis jawapan untuk memastikan jawapan adalah betul, tepat dan persis termasuk penggunaan istilah yang tepat.
- Jawapan mestilah mengandungi fakta yang tepat dan persis untuk mengelakkan salah faham dan salah tafsir.
- Mestilah menguasai semua konsep asas kimia terutamanya konsep mol dan Jadual Berkala Unsur.
- Selalu membuat latihan berkaitan melukis rajah yang berfungsi dan melabelkan dengan lengkap.
- Memahami soalan dengan teliti samada jawapan perlu menggunakan formula atau nama bahan kimia.
- Tuliskan unit yang betul apabila menyelesaikan masalah pengiraan.
- Mengingati simbol kimia bagi memudahkan menulis persamaan kimia yang seimbang.
- Dapat membezakan antara atom, ion dan molekul.
- Boleh membezakan antara siri elektrokimia dengan siri kereaktifan.
- Menggunakan bahasa, istilah saintifik dan tata bahasa yang betul apabila menjawab soalan.
Petikan dari Kupasan Mutu Jawapan Kimia SPM 2010
2013-11-21
Electrolysis of Copper(II) Sulphate
Be analytic when study electrolysis of an aqueous solution.
3 factors affecting product of electrolysis of an aqueous solution.
- type of electrode
- concentration of ion in aqueous solution
- position of ion in the Electrochemical Series.
What factors are affecting product at anode & cathode during electrolysis of copper(II) sulphate solution?
Position of ion in the Electrochemical series
How do concentration & type of electrode affect product of electrolysis?
Arrangement of Atoms in Bronze
2013-10-12
2013-07-15
2013-07-07
Electrolysis of Molten Lead(II) Bromide
 |
| Set-up of apparatus to study electrolysis of molten lead(II) bromide |
| Diagram shows reactions occurs during electrolysis of molten lead(II) bromide at both electrodes |
- Molten lead(II) bromide consists of lead(II) ions and bromide ions.
- During electrolysis of molten lead(II) bromide, bromide ions move to anode and lead(II) ions move to cathode.
- At anode, bromide ions are discharged by releasing electrons to form bromine. Half equation for the reaction is 2Br- --> Br2 + 2e-
- At cathode, lead(II) ions are discharged by accepting electrons to form lead. Half equation for the reaction is
2013-05-18
2013-04-18
2013-04-13
The Making of Margarine
What is the effect on the oil used during the making of margarine?
- The liquid oil changes to solid.
- Relative molecular mass of oil molecules increase.
2013-04-11
2013-04-08
Reaction between Sodium and Chlorine
The video above shows the reaction between sodium and chlorine.
- What is the observation?
- Name the product of the reaction.
- Write chemical equation for the reaction between sodium and chlorine.
2013-04-06
Study Visit to Malaysia Nuclear Agency
A 3 hours study trip indeed have given students and teachers a deeper understanding on uses of radioisotopes in Malaysia context.
2013-03-30
DNA Molecules
DNA stands for deoxyribonucleic acid. It is an important molecules that carry genetic information for living organisms. The molecule forms double helix structure.
Picture Source : Wikipedia
DNA is a polymer that made up of the smaller repeating units, called nucleotides.
Some fun facts about DNA(from 10 Interesting DNA Facts
Fun, Useful, and Interesting Facts about DNA, By Anne Marie Helmenstine, Ph.D., About.com Guidez)
- If you put all the DNA molecules in your body end to end, the DNA would reach from the Earth to the Sun and back over 600 times (100 trillion times six feet divided by 92 million miles).
- A parent and child share 99.5% of the same DNA.
Knowledge about DNA is widely used in the following fields :
- DNA profiling
- Genetic engineering
- History and anthopology
- Bioinformatics
- DNA nanotechnology.
2013-03-27
Isomers of Propanol
Propan-1-ol
Isomerism in Alcohols due to two reasons :
- Position of its functional group, -OH group
- Branching of carbon chains
2013-03-26
How does temperature affect eggs?
Do you like eggs? Half-boiled, fried egg, etc....
Have you ever pondered these questions?
Have you ever pondered these questions?
- Do different temperature affect the texture of eggs?
- Does the size of eggs affect the cooking of eggs?
I thought cooking eggs just a simple thing for every lay man like me. I was surprised to find that a branch of food science, called Molecular Gastronomy investigate all these.
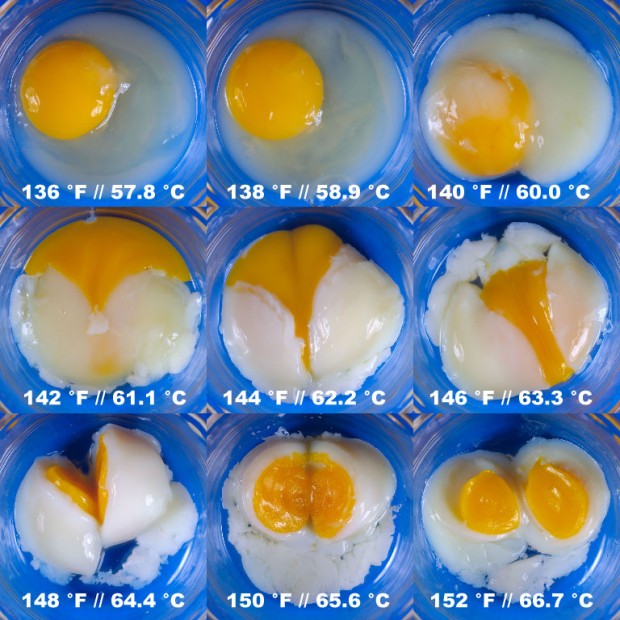
Source : DouglasBaldwin.com
The picture shown eggs cooked in a water bath for 75 minutes at temperature from (57.8 0C to 66.7 0C) by Douglas Baldwin.
In Khymos's blog, Towards the perfect soft boiled egg, I even found a formula relating temperature of water, mass of eggs & cooking time.
Amazing?
According Wikipedia, Molecular Gastronomy is a sub-discipline of food science that seeks to investigate, explain and make practical use of the physical and chemical transformations of ingredients that occur while cooking, as well as the social, artistic and technical components of culinary and gastronomic phenomena in general.
2013-03-19
First Landfill Gas Power Plant
First Landfill Gas Power Plant in Air Hitam Sanitary Landfill, Puchong, Selangor . The park was recognized as first Electrical New Energy Power Plant which uses landfill gas in Malaysia Book of Records.
The power plant generates 2.0 Megawatt electricity supply by trapping and burning a by-product of of decaying waste - Methane gas.
Based on newspaper report, Air Hitam Sanitary Site has received solid wastes collected from Klang Valley since 1995 for 11 years. The site was ordered to closed by the state due to new housing area nearby.
Landfill gas produced from chemical reactions and microbe actions upon the solid wastes. Methane gas is one of the main composition in the landfill gas produced. The gas collected through system of pipes & well are then burned to carbon dioxide, water & heat.
"It is estimated that that there is here is enough gas here to power the generator to provide about 15 years of electricity to about 2,000 households", Worldwide Holdings chairman Datuk Mohd Arif Abd Rahman said.
For further reading, please click
2013-03-17
Chloroform
Trichloromethane or known as chloroform, CHCl3 is one of the product of substitution of methane with chlorine(chlorination) in the presence of UV sunlight. The output of this process is a mixture of chloromethane, dichloromethane, trichloromethane & tetrachloromethane).
The following are the chemical equations that show the step by step reactions in substitution reaction.
Uses of chloroform
- a common solvent
- was once a widely used anesthetic(Its vapour easily depress the central nervous system)
2013-03-11
Determine the Physical State of A Substance at a Given Temperature
SPM 2012 Paper 1 Question 25
- The melting point and boiling point of V and W are lower than 1000C. Both exist as solid at 1000C.
- The melting point of X is lower than 1000C. The boiling point of X is higher than 1000C. It exists as liquid in room temperature.
- Both the melting point and boiling point of Y are lower than 1000C. It exists as gas in room 1000C.
2013-03-08
Avogadro Constant & Amedeo Avogadro
Avogadro Constant :
(Definition : Number of particles in 1 mole of a substance )
is named after
Amedeo Carlo Avogadro(1776 - 1856)
for his great contribution towards chemistry.
2013-03-02
The Meaning of Relative Atomic Mass
A carbon-12 atom is chosen as a reference. Carbon-12 is assigned a mass of exactly 12 unit.
Mass of an atom of an element when compared with 1/12 of mass of a carbon-12 atom.
2013-02-22
Butane in Bunsen Burner in School Laboratory
A common Bunsen burner used in the school laboratory. It consists of butane gas.
The butane gas in the cartridge under combustion in the presence of oxygen gas produce carbon dioxide and water.
Chemical equation for complete combustion of butane.
When there is insufficient of oxygen(the hole of Bunsen burner is closed), incomplete combustion occurs and sooty flame is produced.
2013-02-16
New York Call for a Ban on Styrofoam
What we commonly call Styrofoam is more technically known as foamed polystyrene.
New York City Mayor Michael Bloomberg recently just plans a ban on Styrofoam food packaging from stores and restaurants.
Common uses of Styrofoam
New York City Mayor Michael Bloomberg recently just plans a ban on Styrofoam food packaging from stores and restaurants.
Common uses of Styrofoam
- disposable coffee cups, plates
- cushioning material in packaging
The structure of polystyrene.
2013-02-04
Number of Atoms & Number of Molecules
Which of the following substance contains 1.204 x 1024 atoms?
A 1 mol of nitrogen gas
B 1 mol of ammonia
C 1 mol of water
D 1 mol of argon
Many of my students choose the wrong answer in the current topical test.
1 mol of nitrogen gas consists of 1 x 6.02 x 1023 molecules.
Number of atoms in 1 mol of nitrogen gas is 2 x 6.02 x 1023 .
(Each nitrogen, N2 molecule consists of 2 nitrogen atoms)
1 mol of ammonia consists of 1 x 6.02 x 1023 molecules
Number of atoms in 1 mol of ammonia gas is 4 x 6.02 x 1023.
(Each ammonia, NH3 molecule consists of 4 atoms)
1 mol of water consists of 1 x 6.02 x 1023 molecules
Number of atoms in 1 mol of water is 3 x 6.02 x 1023
(Each water, H2O molecule consists of 3 atoms)
1 mol of argon consistss of 1 x 6.02 x 1023 atoms.
The correct answer : A
A 1 mol of nitrogen gas
B 1 mol of ammonia
C 1 mol of water
D 1 mol of argon
Many of my students choose the wrong answer in the current topical test.
1 mol of nitrogen gas consists of 1 x 6.02 x 1023 molecules.
Number of atoms in 1 mol of nitrogen gas is 2 x 6.02 x 1023 .
(Each nitrogen, N2 molecule consists of 2 nitrogen atoms)
1 mol of ammonia consists of 1 x 6.02 x 1023 molecules
Number of atoms in 1 mol of ammonia gas is 4 x 6.02 x 1023.
(Each ammonia, NH3 molecule consists of 4 atoms)
1 mol of water consists of 1 x 6.02 x 1023 molecules
Number of atoms in 1 mol of water is 3 x 6.02 x 1023
(Each water, H2O molecule consists of 3 atoms)
1 mol of argon consistss of 1 x 6.02 x 1023 atoms.
The correct answer : A
2013-01-30
Examples of Hydrocarbons
The above figure shows different examples of carbon compounds.
As we exam the structure of every molecule, each carbon atom is with bonded with other atoms(carbon atoms/hydrogen atoms) through either single bond, double bond or triple bond.
Which examples shown is saturated hydrocarbons?
Saturated hydrocarbons are Organic compounds containing only carbon & hydrogen atoms only.
2013-01-24
Interpreting Graph relating to Rate of Reaction
Hydrogen peroxide decomposes to water and oxygen.

In this reaction, rate of reaction is measured by the changes in volume of oxygen gas released per unit time.
Rate of reaction can be shown by a steeper curve.
Curve Q has a steeper curve. The rate of reaction is higher. The possible factors affecting the rate of reaction is : size, concentration, temperature, pressure and catalyst. The possible factor mostly is concentration. Higher concentration, higher rate of reaction.
Curve Q shows that volume of gas released is lesser.
Volume of oxygen gas released depends at the quantity of reactant(in more specific, number of moles of hydrogen peroxide used).
Number of moles of hydrogen peroxide used in the initial experiment is
(0.1 x 25)/1000 = 0.0025
After determining number of moles of hydrogen peroxide given in A, B, C & D.
Only the number of moles of hydrogen peroxide in C is the smaller than 0.0025.
The correct answer : C
Determining Rate of Reaction
In the reaction between limestone powder(calcium carbonate) hydrochloric acid, 17 cm3 of carbon dioxide that produced is in 3.5 minutes.
Average rate of reaction is 17 cm3/3.5 min
But, during the second minute, only (12.5 - 7.5) cm3 of carbon dioxide gas released in 1 minute time,
Average rate of reaction during the second minute is
(12.5 - 7.5) cm3 / (2 - 1) min
The correct answer is : C
2013-01-09
Slow Reaction
Slow reaction has low rate of reaction. It takes a long time for the reaction to be complete.
Examples of slow reaction :
Rusting of Iron
An iron takes a long time to react with oxygen and water to form rust(brown solid on the iron nail), which is known as hydrated iron(III) oxide, Fe2O3.3H2O.
Formation of stalagmites
Stalagmites are formed due to the dripping of mineralized solutions and the deposition of calcium carbonate. Normally this stalagmites formation only occurs under certain pH conditions within the underground cavern.

Ripening of fruits
Examples of slow reaction :
Rusting of Iron
An iron takes a long time to react with oxygen and water to form rust(brown solid on the iron nail), which is known as hydrated iron(III) oxide, Fe2O3.3H2O.
 |
| *credit to Wikipedia |
Formation of stalagmites
Stalagmites are formed due to the dripping of mineralized solutions and the deposition of calcium carbonate. Normally this stalagmites formation only occurs under certain pH conditions within the underground cavern.

Ripening of fruits
Subscribe to:
Posts (Atom)

















.gif)





