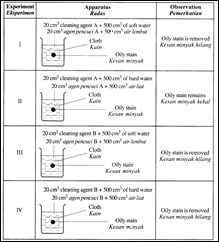
Diagram shows the apparatus used to investigate how to remove an oily stain from a cloth and the observation made from that investigation.
(i) Compare and explain the effectiveness of the cleaning action between
- experiment I and III
- experiment II and IV.
(ii) identify the cleaning agents, A and B. State which cleaning agent is more effective.
Suggested answer
(i) Experiment I and III
- Both the cleaning agents A and B are effective in soft water. Soft water does not consist of calcium and magnesium ions.
- Both dissolves in soft water.
- They are able to lower the surface tension of water. The water wets the surface of the cloth thoroughly.
Experiment II and IV
- Cleaning agent A is not effective in hard water. Hard water consists of calcium and magnesium ions.
- These ions react with the cleaning agent A to form an insoluble precipitate (scum). Formation of scum reduces the number of cleaning agent A molecules available for cleaning.
- Cleaning agent B is effective both in soft water and hard water.
- Cleaning agent B does not form precipitate (scum) in hard water. Cleaning agent B form soluble salt in hard water. The cleaning agent B molecules are available for cleaning.
(ii) Cleaning agent A is soap; Cleaning agent B is detergent.
Detergent is more effective than soap.